Starting a Clinical Trial in Armenia- Pharma/Medical Device/Advanced Therapies
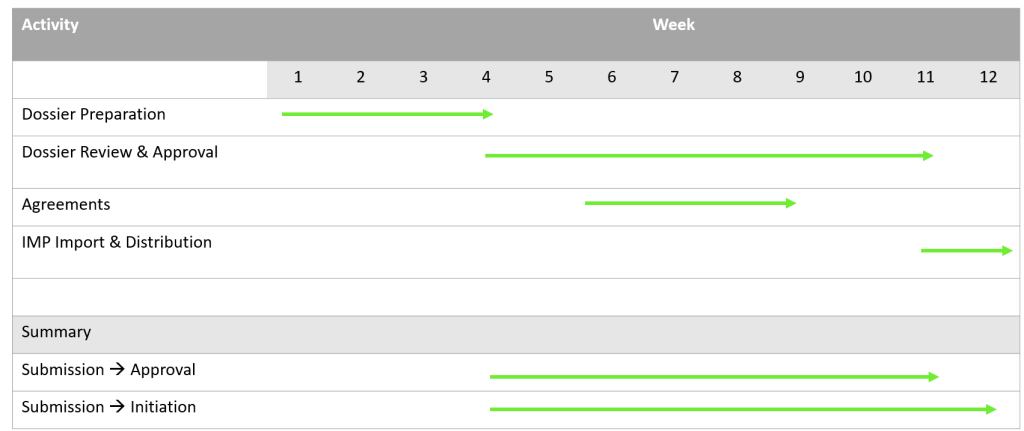
Pharma and Advanced Therapies: First step is arranging the submission dossier which usually takes 3-5 weeks. Then we submit the dossier to the Armenian Ministry of Health (MoH). The review process and study approval takes 40-70 days.
Medical Device: First step is arranging the submission dossier which usually takes 3-5 weeks. Then we submit in parallel to the Central Ethical Committee (CEC) and to the Armenian Ministry of Health (MoH). The review process and study approval takes 4 weeks.
Agreements with study sites and investigators is done in parallel to the regulatory approval process. We can assist you with the agreement negotiations. It usually takes us 2-3 weeks to achieve that.
Last step is to obtain an import permit. This will take another 1 week.
So, summing it all, within 3 months from “green light” you will have your study ready to go.