Starting a Medical Device Clinical Trial in Georgia
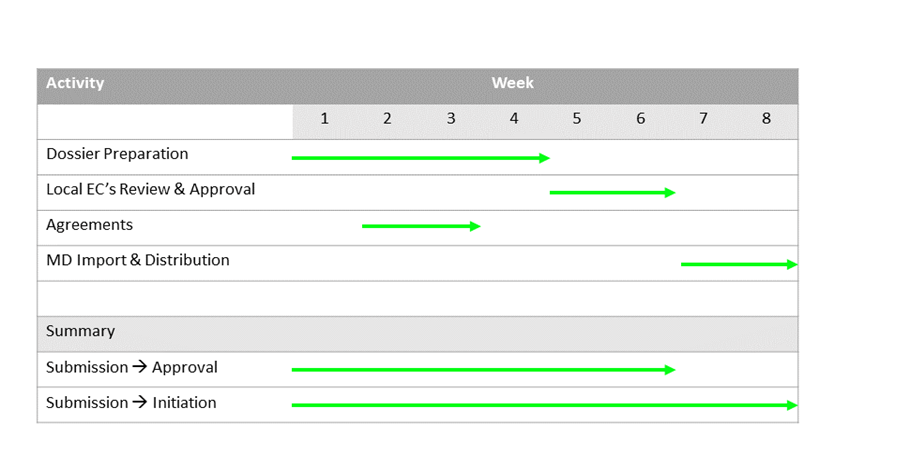
First step is to obtain an approval from the local ethical committees (LECs) from each of the study sites. Arranging the submission dossier usually takes 3-5 weeks and the review process in the LECs takes an additional 1-2 weeks.
No review is needed by the MoH.
Agreements with study sites and investigators is done in parallel to the regulatory approval process. We can assist you with the agreement negotiations. It usually takes us 2-3 weeks to achieve that.
Last step is to obtain an import permit. This will take another 2 weeks.
So, in 2 months you are ready for your first site initiation.